Carbohydrates 1 - Monosaccharides
Chocolate, the one thing we never come to hate (even after being diagnosed with diabetes 😂), have glucose which gives that sweet taste to it. Glucose is a monosaccharide. It is the main source of energy in animals and plants where it is responsible in producing ATP (molecules which provide energy) during cellular respiration which is a series of oxidation reactions (reactions where electrons are donated to outside from that molecule of Glucose).
Let us try to understand about Monosaccharides through this simple sugar.
The Structure of Glucose is shown in the below image,
As you can see, it is a simple organic sugar molecule (Well, not so simple for those who are new to this😁) made of 6C atoms. Therefore it is called a Hexose sugar with -OH and -H groups attached to it.
Since the functional group in this Organic compound is a Carbonyl group (aldehyde group) it is called an Aldose.
In the presence of an Aqueous solution the linear form is in equilibrium with the ring structure as shown above.
The presence of asymmetric Carbons allows the ring structure to form different versions of itself by changing the spacial arrangement of its functional groups.
[Asymmetric C is a C attached to 4 different functional groups]
These structures are referred to as isomers (there are different types of isomers in this case the type of isomer referred to is called Stereoisomer).

The 1st type of stereoisomers we are going to consider is α and β anomers which is formed by a process called Mutarotation. This can be shown in the image below, (pyranose is another name for a hexose ring),
Another way we can look into this is by considering how the
-OH group, attached to the C5 that is adjacent to the terminal alcohol group,
is oriented in the linear form of a Glucose molecule. If, it is oriented to the left
then it is L form and to the right then D form.
In both the above cases, we are looking at the mirror images
of the Glucose molecule. Since these mirror images are non-super imposable, they
are referred to as Enantiomers.
The 3rd example of
stereoisomerism observed is called Epimers. When the orientation of -OH groups in the molecule differ in a single C atom only, forming 2 different molecules,
then those 2 molecules are called epimers of each other. For example, Glucose
and Galactose are epimers of each other since only the -OH group attached to
the C4 of these 2 molecules change their orientation.
Now let us take another example of monosaccharides which we can find present in the honey we eat.
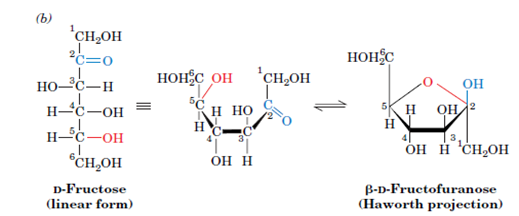
This is called Fructose. It is found in Honey, Table sugar and Fruit juices.
It has the same chemical formula as glucose i.e., C6H12O6 with 6 C atoms but it is sweeter. How is that possible? The answer to that lies in the structure. The structure of a fructose molecule is shown here,
Both Glucose and Fructose are monosaccharides.
They are simple sugars and monomers.
By studying the above 2 examples what can we learn about Monosaccharides?
- The first thing you should have noticed is that they all have Carbon, Hydrogen and Oxygen as their structural components. This is true in every Carbohydrate ( in addition to that Sulphur, Phosphorous and Nitrogen can be seen in Polysaccharides).
- 2 types of principle functional groups can be seen in monosaccharides. They are, Aldehyde and Ketone groups( Carbonyl Groups). Based on this, monosaccharides can be divided in to 2 main categories called Aldoses an ketoses.
- The presence of these Carbonyl Groups allows them to undergo reactions that will form ring structures. 5C and 6C sugars in the presence of an aqueous medium form ring structures which are stable while that of 3C, 4C and 7C sugars are unstable.
- Monosaccharides can be divided based on the number of Carbon atoms. Accordingly, we can categorize monosaccharides as shown,
Based on the number of C atoms in the chain the following
prefixes are used to name a monosaccharide,
·
3C – Tri
·
4C – Tetra
·
5C – Penta
·
6C – Hexa
·
7C – Hepta
They all end with the suffix “-ose” when they are
used in naming sugars.
They
have a general empirical formula of [ C . H2O]
n.
n
= the no. of C atoms in the
molecule
n ≥ 3

6. By studying these sugars, you can see that the C:H:O ratio
is always 1:2:1 in monosaccharides
Functions
· These monosaccharides have the ability to form covalent bonds with each other ( which will be discussed deeply in the next article). The anomeric group involve in a condensation reaction with alcohol groups forming α and β glycosidic bonds (Greek: glykys meaning sweet.) Thereby they are able to form complex carbohydrates such as cellulose, starch, and glycogen.
- Pentose sugars such as Ribose involve in forming other biological molecules such as DNA (Deoxyribonucleic acids), RNA (Ribonucleic acids) and Ribulose involve in forming Ribulose Bisphosphate which is an important molecule in Photosynthesis.
· Glucose act as the main sugar molecule that act as the main source of energy in animals and plants when it undergoes cellular respiration which is a series of oxidation reactions.
For video lessons on this topic head to Carbohydrate - Monosaccharides.













Comments
Post a Comment