Carbohydrates 2 - Disaccharides Ⅱ
Other examples of Disaccharides are,
1. Maltose
2. Lactose
Glucose + Glucose → Maltose
Functions
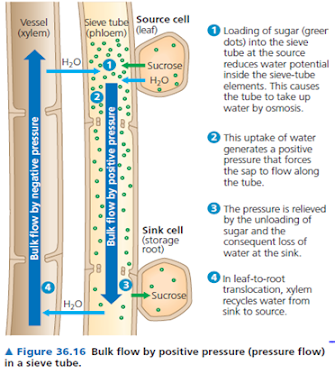
· 1. Sucrose acts as the transporting medium in plants (during phloem translocation).
· 2. Lactose is found in milk and it act as a great source of energy and nutrients.
· 3. Maltose is found in germinating plant seeds and act as a source of energy for the growing plant.
Testing for Reducing and Non-reducing sugars - the test which we can use to identify the presence of monosaccharides, disaccharides and polysaccharides.
Stable monosaccharides such as pentoses and hexoses (furans and pyrans) they exist in an equilibrium in which their linear form is in equilibrium with its cyclic form. Which is a result of the reaction between the hydroxyl group and carbonyl group.
Therefore, free aldehyde groups are able to reduce oxidizing agents. These monosaccharide molecules all of which have free anomeric Carbon act as reducing sugars. (
Similarly, disaccharides with free anomeric C/ Carbonyl C which are not involved in forming glycosidic bonds i.e., which are not free, acts as reducing sugars. Therefore, Maltose and Lactose are reducing sugars while sucrose is a Non-reducing sugar.
Using this concept, we can create a test to identify reducing and nonreducing sugars. Here we use Cupric Sulphate in an alkaline solution called the Benedict’s reagent. (Blue in color)
1. We take a sample of our sugar into a test tube.
2. Add Benedicts reagent and heat it in a water bath over a Bunsen burner.
3. If the color of the solution turns from Blue – green – yellow – orange – Brick red then that contains the presence of a reducing sugar
The formation of a brick red precipitate is due to,
Cuprous Sulphate is insoluble and therefore precipitates.
If such a change in color isn’t observed this implies the presence of a non-reducing sugar.
4. Add a solution of Conc. HCl to the above solution a heat it and add NaOH to neutralize the solution.
Why neutralize the solution by adding NaOH?
The Cupric Sulphate for it undergo the above reaction it needs to be in an alkaline solution and not an acidic solution.
5. Then add the benedict’s reagent and repeat steps 1 – 3.
· If a brick red precipitate is observed after heating with Conc. HCl, it confirms the presence of a Non-reducing sugar.
















Comments
Post a Comment